Meet the Westchester Firm that Saved the President's Life
News Based on facts, either observed and verified directly by the reporter, or reported and verified from knowledgeable sources.
Understanding the science and business behind Regeneron’s COVID-19 biotech breakthrough
By Sherrie Dulworth
Last January, a blip on the viral radar went unnoticed by much of the world. By the time the public became aware of an emerging pestilence—soon to be known as COVID-19—infectious disease researchers at Tarrytown-based Regeneron Pharmaceuticals, Inc., were already on the hunt for new drugs that could combat the disease.
Dr. Alina Baum, Associate Director for Infectious Diseases at Regeneron, is a trained virologist who has been with the company for almost six years and leads a 12-person research team. She recalled the urgency felt in the wake of the rising numbers COVID cases, hospitalizations, and deaths. “In New York in April, it was terrifying,” she said. “Some of the people in my group were working 80-hour weeks trying to get a drug to the clinic.”
In February, the company started laboratory research; by June, their first human clinical trials testing treatment against COVID-19 were underway.
In October, then-President Donald Trump was diagnosed with COVID-19. He was whisked from the White House to Walter Reed Medical Center, where he spent four days and received an investigational medicine made by Regeneron.
Following his release from the hospital, Trump shared a video lauding the treatment while conflating it with the company name and thrusting the biotech company into the world spotlight. He said, “I went in, and I wasn’t feeling so hot. And within a very short period of time, they gave me Regeneron.” After referencing the company name a couple more times, he added, “It was like, unbelievable. I felt good immediately.”

WHAT IT IS, WHAT IT ISN’T, AND HOW IT WORKS
REGEN-COV™ is the brand name for the drug given to the former president, and now hundreds of thousands of other people. It is a combination of two monoclonal antibodies.
Humans naturally produce a protein in our blood known as antibodies that help us fight infection and foreign substances. Monoclonal antibodies are created in a laboratory and are used to treat various diseases. In the case of REGEN-COV, the target is the SARS-CoV-2 coronavirus.
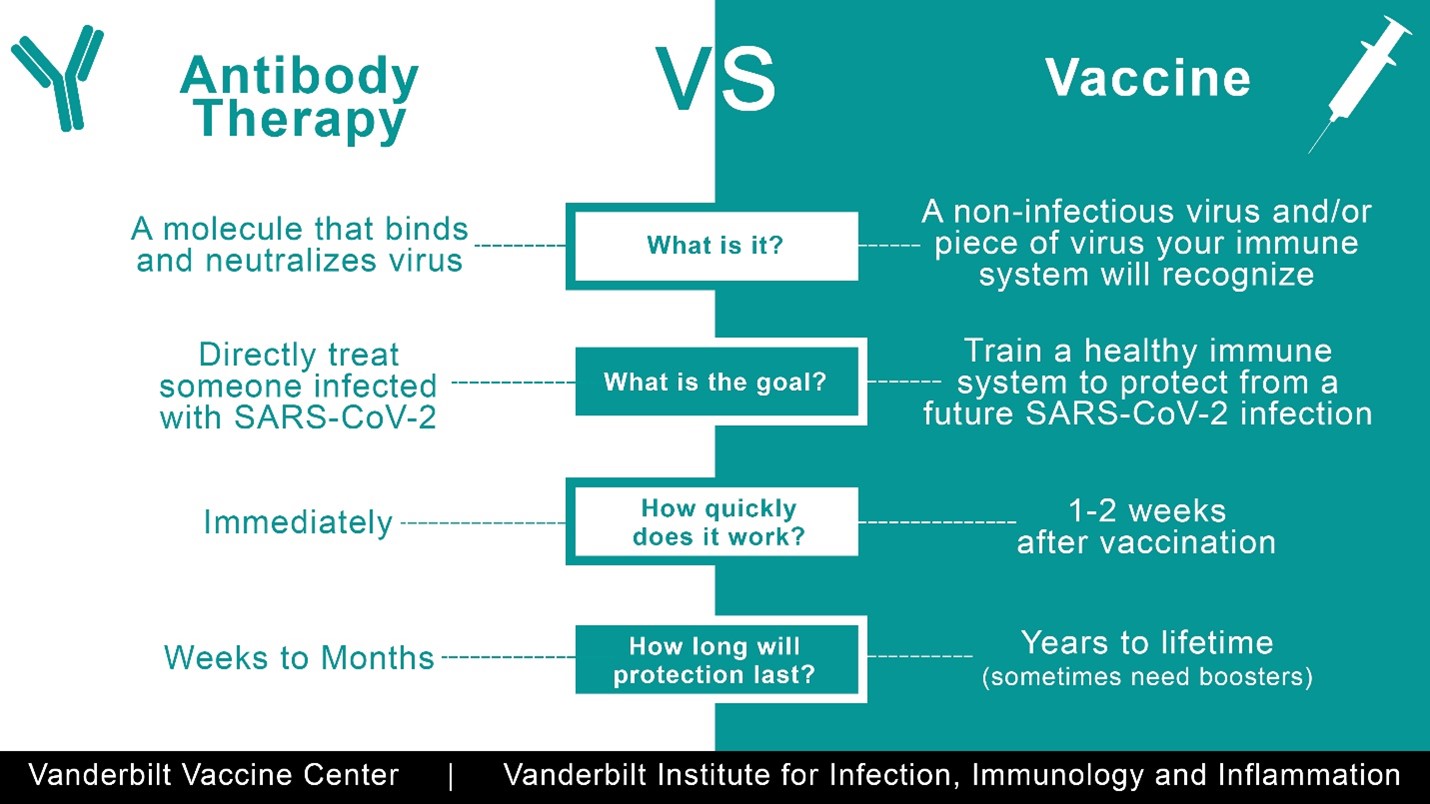
Monoclonal antibodies differ from vaccines, which stimulate your body’s natural immune system to make antibodies that protect you from an infectious disease. (See sidebar for more on how they compare to vaccines.)
Regeneron uses genetically engineered mice that can make thousands of 100-percent human antibodies. Scientists then screen for and select the antibodies that work best against a specific virus. The company’s technology platform dramatically reduces the traditional drug discovery and development timeline. “A process that normally takes months has been reduced to weeks,” Baum explained.
While these specific COVID-19 targeted treatments are new, Regeneron’s technologies to build them date back to 2003. Founded in 1988, the company has launched seven other medicines besides REGEN-COV, including a triple antibody cocktail that has been effective against the deadly Ebola virus.
Like with any medical treatment, REGEN-COV has limitations for its use. It is currently authorized for adults and some children who have mild-to-moderate COVID-19, but not for those who are hospitalized or who require oxygen therapy due to the virus.
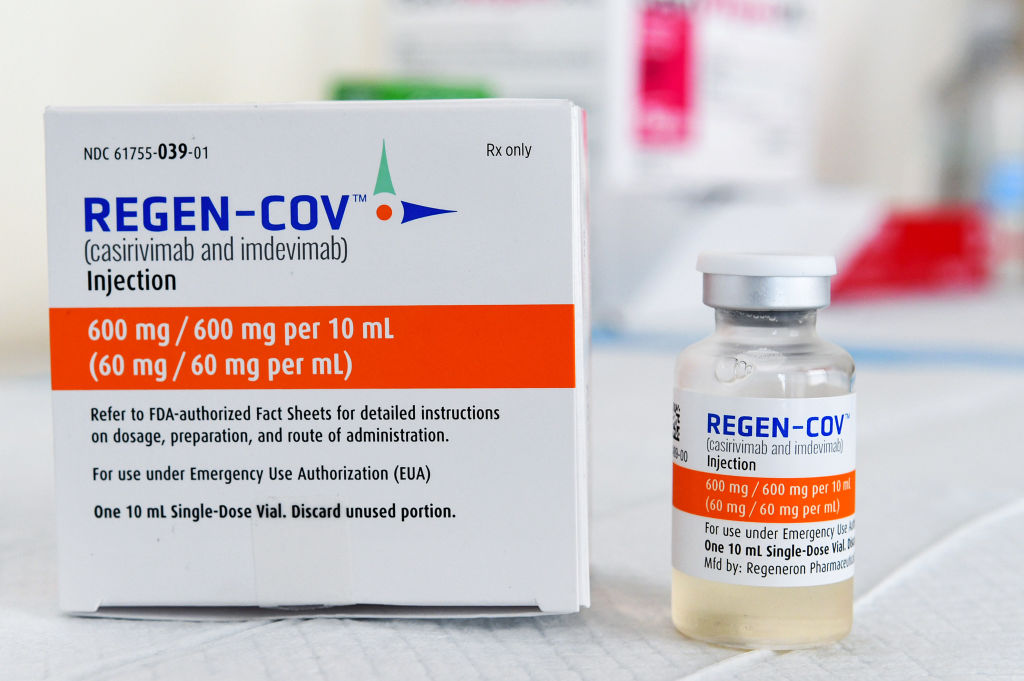
FROM THE PIPELINE TO PEOPLE
The process for a drug to receive full U.S. Food and Drug Administration approval is lengthy. Still, under a declared public health emergency–like in the case of a pandemic–the FDA can make drugs available that haven’t yet been approved.
Last November, the FDA issued an emergency use authorization that allowed REGEN-COV to be used to treat certain high-risk individuals. The EUA was recently expanded to include preventative treatment after exposure to the virus, but not as a substitute for vaccination.
To bring new drugs to market, pharmaceutical companies need boots-on-the-ground clinicians who help with clinical trials. White Plains Hospital was an early participating medical partner in monoclonal antibody research and then in administering the outpatient therapy when it was approved.
Dr. Neritan Mani, Associate Medical Director at White Plains Hospital, oversees their monoclonal program. Although the hospital is not a traditional academic center, Mani explained that their doctors volunteered their free time to assist in research after the first COVID wave. “The doctors were desperately looking for a therapeutic weapon to use against COVID,” Mani said.
During a four-month period, the hospital treated more than 600 patients using Regeneron’s drugs and another monoclonal antibody product made by Eli Lilly & Co., which were approved for emergency use. In a recently released report, they cited that 93 percent of those treated were able to avoid being hospitalized.
Trump is not the only prominent politician to receive REGEN-COV. Earlier this month, 63-year-old Texas Gov. Greg Abbott tested positive for the COVID-19 virus. His office reported that he is receiving Regeneron’s monoclonal antibody treatment.
We are now seeing new COVID-19 surges due to variant strains, or mutations, of the virus. Baum said that the data show that REGEN-COV is proving effective against those emerging variants. “We knew the virus was going to change because that is what viruses do,” she said. “The way we designed our drug was specifically aimed at minimizing the risk that a variant would pop up and the drug would stop working.”

THE BIOTECH AND BUSINESS
Regeneron has also led to business and economic advances, particularly in Westchester County, which represents about 20 percent of the biotech workforce in New York State. The county now has seven biotech therapeutics firms.
About 4,000 of Regeneron’s employees work in Westchester County, or close to 50 percent of their workforce.
According to Bridget Gibbons, Director of Westchester County’s Office of Economic Development, “At the direction of County Executive George Latimer, my office has had a very strong focus on growing the biosciences sector. Because of Regeneron’s foundational presence, we have lots of credibility in attracting start-ups.”
Regeneron has spent the past six years on Fortune magazine’s “100 Best Companies to Work For.” Asked what in the company culture contributes to such consistently high ratings, a company spokesperson replied, “Ours is a different biotechnology company—one that combines cutting-edge biology with a drive for science and technology innovation. Scientists and non-scientists alike share an ambition for unlocking the next scientific breakthrough.”
In July, New York Governor Andrew Cuomo announced that Regeneron intends to invest $1.8 billion to expand its Mid-Hudson footprint, adding new facilities and about 1,000 jobs through the next five years. Gibbons said that along with adding those 1,000 direct new jobs–an estimated 300 of them in Westchester– the project is projected to create 1,600 new construction jobs.
SEEING THE PERSONAL IMPACT
Asked how the researchers feel when they see the impact of their work on individual lives, Baum said it feels a little bit surreal. “You can look at data and numbers, but I think when it really hits is when you read about individual patients. That’s when it touches you on the human level.”
Baum recalled seeing a picture of a mother and baby in the eastern Congo who survived Ebola after receiving Regeneron’s monoclonal antibody treatment. More recently, the team has read letters from people who credit REGEN-COV for saving their loved ones.
“It just brings it home that what you are doing is real, and that it makes a difference,” she added.
SIDEBAR: VACCINES VS ANTIBODY THERAPIES FOR COVID-19
Sherrie Dulworth is a freelance writer based in Westchester County whose writing covers a wide range of stories about healthcare, career development, being a bibliophile, and human interest. When she is not writing, she loves reading—just about everything.

Examiner Media – Keeping you informed with professionally-reported local news, features, and sports coverage.
